Article Text
Abstract
Objectives We used objective assessment tools to detect subtle neurological deficits that accompany repetitive and mild head impacts in contact sport across a season.
Methods Female participants (n=13, 21±1.8 years old; 167.6±6.7 cm; 72.8±6.1 kg) completed assessments pre and post the varsity rugby season. A commercial balance board was used to assess static balance and response to dynamic postural challenge. Spinal cord excitability via the soleus H-reflex was assessed in both legs. Video analysis was used to identify head impact exposures.
Results A total of 172 potential concussive events were verified across 11 athletes (15.6±11; 95% CI: 6.5 to 19.8). Balance performance was worse at post-season for total centre of pressure which increased by 26% in the double stance on a stable surface (t(12)=-2.33; p=0.03; d=0.6) and by 140% in the tandem stance on a foam surface (t(12)=-3.43; p<0.01; d=0.9). Despite that, dynamic postural performance was improved after the season (p<0.01). Spinal cord excitability in rugby athletes did not change across the season but deviated from normative values at baseline.
Conclusion Quantitative measures revealed that exposure to impacts across a competitive rugby season impair balance in two specific stances in female rugby athletes. Tandem-leg stance on an unstable surface and double-leg stance on firm surface are useful assessment conditions when performed over a low-cost balance board, even without clinically diagnosed concussion.
- brain
- concussion
- rugby
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What are the new findings
A competitive rugby season induces subtle deteriorations in neuromuscular function that is not captured in traditional sideline assessment.
Differentiation between static stances which range in difficulty is relevant to uncover subtle changes in balance.
Tandem-leg and double-leg static stances are sensitive to detect centre of pressure alteration following a season of recurrent head impacts.
Spinal cord excitability measurements suggest deviated values at baseline.
How might it impact on clinical practice in the future
Implementing an objective balance measure as a clinical assessment may uncover subtle neurological impairments without diagnosed concussion.
Double-leg stance is often overlooked by subjective assessments, but it provides an insightful outcome if performed using a sensitive tool.
The challenging tandem-leg stance over a foam pad notably contributes to a clinical assessment after recurrent mild head impacts.
Spinal cord excitability may be suitable for detecting particular neurological patterns in female athletes exposed to head impacts.
Introduction
While explorations of ‘visible” concussive incidents are receiving increased attention, the cumulative effects of impact forces that operate below the threshold necessary to produce overt symptoms remain relatively neglected1–4 and inconsistent.5–7If repetitive, mild head impacts may result in deleterious effects to the integrity and function of the brain,1 6 7 neuropsychological vulnerability,8 and vestibular function.9 However, conclusions may differ when standard clinical measures are applied.5
A common practice for evaluating integration deficits of the somatosensory, visual and vestibular systems is the use of balance tests.10 11 For instance, the Balance Error Scoring System (BESS) is a useful sideline assessment tool, but results usually depend on a subjective error counting system and testing conditions.10 12Alternatively, the use of a low cost commercially available balance board (BB) is promising for improving test accuracy and reliability,13 and to quantify dynamic postural adjustments.14 The Hoffmann (H) reflex is also a reliable method to analyse modulations of spinal excitability,15 16 and is sensitive for detecting effects caused by head trauma17 and spinal cord injury.18 In addition to traditional amplitudes, bilateral reflex fluctuations have provided information on (ab)normal states associated with the spinal cord circuitry.19
There is a need to further explore the abovementioned methods as a probe to identify otherwise asymptomatic brain trauma and injury, mainly in contact sports athletes. Rugby is a modality with frequent head impacts due to some techniques (eg, tackle and ruck), and the early identification of disruption can prevent further damage.1 20 Therefore, the aim of the present study was to investigate subtle underlying neurological deficits that may accompany recurrent mild head impacts in female rugby athletes. It was hypothesised that the test battery used would reveal changes in neuromuscular function after a season’s worth of head impacts.
Material and methods
Participants
Female collegiate varsity rugby athletes (n=13; 21±1.8 years old; 167.6±6.7 cm; 72.8±6.1 kg) with 6±2.7 years of rugby experience participated in the study. Inclusion criteria were absence of chronic disease, neurological impairments and acute lower limb injuries affecting balance. The number of previous medically diagnosed concussions ranged from 0 to 3 (mode=1) and eight participants reported sustaining at least one episode. No concussion was diagnosed throughout this study. Participants completed assessments of static and dynamic balance, and soleus H-reflexes before (pre) and after (post) the six games, 12 weeks, varsity rugby season. The athletes provided written informed consent to participate.
Control procedures
A multiple baseline, which included three pre-season visits, within-participant control design was performed as in recent intervention studies.21–24 This approach allows participants to create a reliable pre-season baseline and enable them to act as their own control, thus reducing the impact of between subject variability and allowing for single participant statistical analysis.24 Additionally, although more labour intensive and time consuming, this procedure provides higher internal consistency of measures and decreased variability.21–24 The order of test administration, time of the day and other environmental conditions were consistent for all participants and testing sessions. A video analysis frequency chart for all season games was used to quantify head impacts for each participant.
Balance tests
A commercially available BB (Nintendo, Kyoto, Japan) was interfaced with a computer using customised software (LabVIEW 2011 National Instruments, Austin, Texas, USA), and data were sampled at 100 Hz.25 The BB is a validated (r=0.99) and reliable (ICC=0.88) force detecting instrument that is widely available and relatively inexpensive.13
During the static balance task, participants performed one trial of 20 s of each stance of the BESS (ie, double-leg, single-leg and tandem-leg).10 All three stances were performed barefoot, eyes closed, hands on the hips on the BB board (firm condition) and repeated with a medium density foam (Airex Balance Pad Elite 81002, 50.08 cm x 40.64 cm x 6.35 cm) placed over the BB (foam condition). Centre of pressure (CoP) was defined according to Winter26 and calculated from the BB output for the medio-lateral (CoPML) and antero-posterior (CoPAP) axes, as well as the total path length (CoPT) (online supplementary appendix 1).
Supplemental material
In the dynamic assessment, each participant stood barefoot on the BB, feet at shoulder width, eyes opened, hands on the hips, in front of a laptop screen at chest height which displayed the CoP as a white dot. On the initiation of a trial, a target dot (red) appeared on the screen and moved in random sequence among eight cardinal and ordinal directions. Participants were instructed to shift their weight on the BB so that the CoP met the target on the screen as quickly and accurately as possible. Following the familiarisation set, participants completed five trials with appropriate rest time between them.14 The averaged time to reach target (tTarget), time to return to centre from target (tCentre) and their computed sum (tTotal) were obtained.14
Spinal cord excitability
Participants were seated with both feet flat on the floor. Electromyography was collected using bipolar surface electrodes placed bilaterally on the tibialis anterior, vastus lateralis and soleus muscles while a grounding electrode was placed over the right and left patella. Recordings were sampled at 2.5 kHz, amplified (500 times for soleus and 5000 times for other muscles) and filtered (10 to 1000 Hz for soleus and 100 to 300 Hz for others) (P511 Grass Instruments, Astro-Med, Inc, West Warwick, Rhode Island, USA).
A square wave (1 ms) electrical stimulus was applied simultaneously to both tibial nerves using a Digitimer (Mendtel, New South Wales, Australia) constant current stimulator. Initially, a recruitment curve was recorded where the stimulus intensity was continuously increased until at least three maximal M-waves were recorded.27 Bilateral H-reflexes were then evoked pseudorandomly (1 to 3 s) and the stimulation was adjusted to elicit waveforms between 50% and 80% of Hmax recorded on the ascending limb of the recruitment curve.16 Five hundred and ten sweeps were collected on both legs simultaneously and the first 10 were discarded to remove the initial transient caused by homosynaptic depression.19 28 Coefficient of variation (ie, ratio between SD over the mean) was calculated for the remaining 500 responses.28 The cross-covariance (CCV) of H-wave amplitude sequence was evaluated using the same method developed by Mezzarane and Kohn.19
Head impact video analysis
Two independent reviewers analysed a total of six games, obtained from videos recorded by the University women’s rugby team. The reviewers were former athletes who were knowledgeable of the rugby rules and trained to recognise the visible signs of concussion, consistently described elsewhere.29 Both reviewers detected impacts in which impulsive forces resulted in whiplash type head movements during a game (eg, tackled from front, tackled from behind, ruck). Within each of the impacts registered, the player was further observed for visible signs of concussion. A potential concussive event was registered when both reviewers independently agreed after analysing the visual signs.
Statistical analysis
All statistical analysis was performed using SPSS (V.24, Armonk, New York: IBM Corp) with the level of significance set at p<0.05. For group comparisons, the three pretesting sessions were first compared via Repeated Measures Analysis of Variance. Sphericity was assumed via Mauchly’s test (p>0.05) and if not, df and p values were corrected using Greenhouse-Geisser method. Given that there was no difference between the pre results, an averaged preseason value was formed and then compared with the post season result using a paired t-test. For individual comparisons, a 95% CI was established from the three prevalues. Post values were then compared with the respective 95% CI, and considered statistically significant if they fell outside the range.24 Independent t-tests were conducted to compare the coefficient of variation and the CCV from the present study with original data from Mezzarane et al.28 Assumptions of normality were confirmed via Shapiro-Wilk test. Effect size (Cohen’s d) was calculated to provide magnitude of changes.30 Data from the head impact video analysis are presented using descriptive statistics.
Patient and public involvement
No, patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research
Results
Static balance assessments and head impacts
Results from the analysis of variance showed no difference between the three baseline tests in the firm and foam surfaces, respectively, for the double-leg CoPML (F2,18=0.67, p=0.5; F2,20=0.39, p=0.6), CoPAP (F2,18=0.66, p=0.5; F2,20=1.64, p=0.2), CoPT (F2,18=3.87, p=0.06; F2,20=0.34, p=0.7), single-leg CoPML (F2,18=1.85, p=0.1; F2,20=0.19, p=0.8), CoPAP (F2,18=0.68, p=0.5; F2,20=0.13, p=0.8) and CoPT (F2,18=1.92, p=0.1; F2,22=0.11, p=0.9) and tandem-leg stance CoPML (F1,10.5=0.87, p=0.3; F1.3,13=0.7, p=0.4), CoPAP (F1,10=0.98, p=0.3; F1.2,12.3=0.95, p=0.3) and CoPT (F1,10.4=1.22, p=0.3; F2,20=1.29, p=0.3).
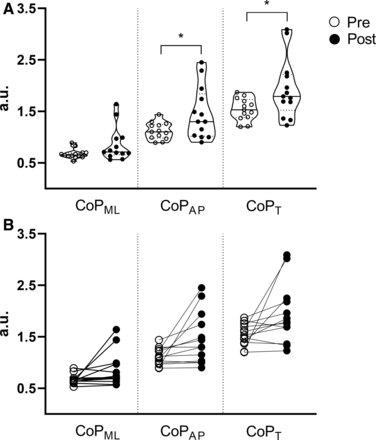
On the firm surface, group analysis showed differences in the CoPAP (t(12)=-2.56; p=0.02; d=0.7) and CoPT (t(12)=-2.33; p=0.03; d=0.6) and on the double-leg stance (figure 1A). There was no difference in the CoPML in the double-leg stance, nor in CoPML, CoPAP and COPT in the single-leg and tandem-leg stances (p>0.05). Individual analysis showed that five and six participants worsened performance in the CoPAP and CoPT, respectively (figure 1B).
Pre–Post comparison of group (a) and individual (b) results of medial-lateral (CoPML), anterior-posterior (CoPAP), and total (CoPT) centre of pressure in the double-leg stance on a firm surface. *p<0.05.
Over the foam surface, individual analysis showed that performance for the majority of participants (seven for CoPML and CoPAP; nine for CoPT) significantly worsened in the tandem-leg stance at the end of season (figure 2B). Changes were 180, 160, and 140% for the CoPML (t(12)=-2.86; p=0.01; d=0.8) CoPAP (t(12)=-2.55; p=0.02; d=0.7), and CoPT (t(12)=-3.43; p<0.01; d=0.9), respectively (figure 2A). There was no difference in the CoPML, CoPAP, and CoPT in the double- and single-leg stances (p>0.05).
Pre-Post comparison of group (a) and individual (b) results of medial-lateral (CoPML), anterior-posterior (CoPAP) and total (CoPT) centre of pressure in the tandem-leg stance over a foam surface. *p<0.05; **p<0.01.
Eleven out of 13 (84.6%) participants accounted for a total of 172 impacts considered as potential concussive events throughout the six games season. For each athlete-exposure (ie, one athlete in one game), 2.2 potential concussive events were registered, with a minimum of 2 and maximum of 34 events per participant (mean±SD: 15.6±11; 95% CI: 6.5 to 19.8; median: 20; mode: 20). The two athletes that did not present any potential concussive event during the season games were further investigated. Performance in the double-leg firm was maintained (CoPML and CoPAP) and increased (CoPT) in one athlete and worsened in the other. In the tandem-leg foam, both athletes’ performance worsened in all variables.
Dynamic postural assessment
Analysis of variance showed no difference between the three baseline tests for tTarget (F2,20=8.73, p=0.05), tCentre (F1.1,12=3.96, p=0.07) and tTotal (F1,10.4=4.79, p=0.06). Group analysis showed that tCentre (t(12)=3.48; p<0.01; d=1.2) and tTotal (t(12)=4.19; p<0.01; d=1.2) were decreased (ie, improved performance) in the post season (figure 3A). Individual analysis suggests that performance was improved by seven and maintained by six athletes (figure 3B). No difference was shown in tTarget (t(12)=2.15; p=0.05; d=0.5), where four athletes improved, six maintained and three worsened performance.
Time to reach the target (tTarget), to get back to centre (tCentre) and the sum of them (tTotal) in the dynamic postural test; group (a) and individual (b) pre versus post analysis. *p<0.05.
Spinal cord excitability
Reflex amplitudes (normalised to supramaximal M-wave amplitudes) were not significantly different from pre to post, confirming that our measures were well controlled in the different time points of data collection. Pre-season left and right Hmax/Mmax ratios were 54% and 63%, while post-season values were 42% and 45%, respectively. Pre-season left and right H-reflex amplitudes were 46±11.6 and 48.9%±13% of Hmax (32.4±21.7 and 26.4%±18.9% of Mmax, respectively), while post season amplitudes were 65±14 and 65.6%±14% of Hmax (31.6±14.7 and 27.3%±15.1% of Mmax, respectively). There were no statistically significant changes in H-reflex amplitude for left (p=0.67) or right (p=0.29) legs. M-wave amplitudes on the left and right sides were 6.2±7 and 6.2%±9.4% of Mmax in the pre-season, while in post season amplitudes were 6.9±10.2 and 8.3%±7%, respectively.
No difference was found between time points in the coefficient of variation for the right (pre: 41±28 vs post: 32%±21%; p=0.31, d=0.3) and left legs (pre: 42±33 vs post: 31%±17%; p=0.27, d=0.2). Likewise, the CCV was unchanged from pre (0.5±0.13) to post (0.53±0.16) (p=0.54, d=0.2).
To assess relative spinal cord excitability in this sample of contact sport athletes, pre-season baseline values from the present study were compared with original data on recreationally active (non-contact sport) participants from Mezzarane et al,28 and results are shown in figure 4. The CCV was significantly higher in the present study (p<0.001, d=2.63), whereas no difference was observed for the coefficient of variation of the right (p=0.16, d=0.58) and left legs (p=0.1, d=0.67).
Comparison between non-contact control population from Mezzarane et al (2017) with the pre-season data of our sample. Mean±SD of cross covariance (CCV) (left Y axis) and coefficient of variation (%) for right and left legs (right Y axis).*p<0.001
Discussion
Balance performance in double and tandem stances are impaired across a season of impact exposures
Our data suggest that tandem stance on an unstable surface is the most susceptible static balance measure for revealing subtle postural decrements given the noticeable worsening of CoP displacement from pre-season to post season. Moreover, there was a CoP increase between study time points of double-leg stance on a firm surface. Our findings highlight the sensitivity of the BB instrument by presenting deficits associated with both a challenging stance (ie, tandem), but also the easiest one that often gets overlooked (ie, double-leg) given its status as a fundamental posture for human locomotion development.31
Despite being a practical tool, the BESS lacks of discrimination between increasingly difficult balance stances, with the analysis based on total score.10 Therefore, successful performance of less challenging postures or learning effects in serial administration of assessments may mask the subtle changes to balance that we observed here. For instance, the double-leg stance in a firm surface is the only stance where a perfect score (ie, no errors) is often achieved, making it impossible to assess its reliability10 and validity.13
The integration of a low-cost commercially available tool to a well-known balance test protocol was able to unveil subtle changes in two stances after recurrent mild head impacts. Conversely, Murray et al5 did not observe impairment in postural control assessed with sensitive linear and non-linear metrics over the course of a competitive football season, although a different testing protocol was performed. Studies using advanced neuroimaging techniques were able to detect impairments after repetitive head impacts,6 7 32 including correlation of head impacts throughout a season with microstructural alterations of grey matter7 and dynamic cerebral autoregulation.6 In these studies, head impacts were assessed through sensors during practices and games. Further analysis in the present study showed that, even with no potential concussive event recorded during the games, two athletes exhibited worsened performance in most of the variables, highlighting the importance of tracking impacts in practices.
Time in dynamic postural test improves in athletic population
Assessing postural control is an indirect way of uncovering neurophysiological damage to supraspinal centres after suspected concussion.33 It was expected that a season’s worth of head impacts would be reflected in task incompetency in a dynamic balance test where attention must be divided between simultaneous cognitive and motor functions.34 Also, the competition between cognitive and motor resources has been shown to persist up to 1 month following a concussion,35 whereas exclusively neurocognitive tests seem to be insensitive in female athletes.1 36 However, there was a decrease of 20% in the time it took for participants to return to the centre and 14% in the total time after the postural challenge between study time points.
Results indicate skill improvement in the dynamic postural test. Our sample was composed of competitive athletes skilled at performing object tracking,37and with an inherent motivation for the task, a potential confounding factor also seen in other athlete studies.38 39Despite any observable task ineptitude here, computerised dynamic postural assessment remains a potential area to explore for assessing neurophysiological decline with accumulated head impacts. However, it must be taken into account to ensure the absence of learning effects, as demonstrated here by the absence of difference among three baseline tests, and consider that neurophysiological changes from repetitive impacts may precede discernible neurocognitive deficits.1
Spinal cord excitability measures are a useful adjunct
Results of coefficient of variation for both legs and CCV did not change significantly from pre to post despite a season’s worth of accumulated head impacts. While the first represents the variability of the H-reflex amplitude sequences in each leg, the latter enables the detection of bilateral fluctuations in H-reflex excitability and exposes temporal linkages between right and left legs.28 Both techniques have been used mostly in neurologically intact population,19 28 40 whereas the rugby athletes in this study have a history of exposure to body impact and head trauma, including concussive episodes, which could be presumed to affect descending spinal cord regulation. Prior work associated H-reflex modulation with the descending control from supraspinal centres18 thus motivating the direct comparison of two distinct samples in terms of exposure to head trauma (ie, the present data with Mezzarane et al).28
Female athletes from the present study showed significantly higher values of CCV compared with the neurologically intact participants from Mezzarane et al.28 This may suggest that the cumulative impact of rugby training and playing alters supraspinal regulation of bilateral fluctuations in spinal cord excitability. Such a chronic alteration as a potential result of athletic training and impact exposure may drive a neuroplastic change in CCV, thus rendering it insensitive to further change across a competitive season. Nonetheless, factors as methodological procedures15 16 and chronic joint instability41 are known to influence spinal cord excitability and should be considered, especially the latter in an athletic population. Limited but unfortunately flawed evidence suggests that some athletes may present higher values of H-waves CCV.40 Significant methodological issues and interpretation within the prior study of Ceballos-Villegas et al40 prevent firm conclusions for their own data and impair translation to ours. This is an area in which further investigation is needed to translate how an increased CCV reflects the evaluation of plastic changes in the motor reflex responses in humans. Although approximately 50% higher, results of coefficient of variation from female rugby athletes were not different compared with neurologically intact population.28 However, the observed effect sizes for the right and left legs justify future studies to verify whether a longer history of head impact exposure can affect this measure. It was already shown that neurologically intact subjects presented more synchronous Ia afferent input between legs compared with individuals with spinal cord injury.18
Although insensitive to detect differences as a result of recurrent head impacts over a season due to chronic pathological adaptation indicated above, bilateral H-reflex amplitude fluctuations seems promising to reveal particular neurological patterns in individuals with years of accumulated exposure to head impact. Full exploration of this hypothesis was beyond the scope of this project but warrants future consideration. There are evidence of injury in the central nervous system despite no overt behavioural deficits following head impacts,4 and that head injury affects many areas of the nervous system, not just where the mechanical stress is applied.17 Alternatively, H-reflex changes may be suitable for symptomatic athletes as there is likely greater damage to supraspinal control centres in the acute phase following a head trauma.17
Some important limitations should be identified in the present study and considered in future investigations. Impacts to the head during practices were not tracked, which would have enriched the analysis possibilities and is suggested as a key focus point in future studies. Also, musculoskeletal injuries resulted from the season were not considered, although acute lower limb injuries affecting balance was considered an exclusion criteria. Literature has endorsed that trials longer than 20 s are needed for CoP measures and different testing protocols may be applied. Additionally, it is important to consider that a change in a particular plane of movement (eg, CoPAP) is likely to have a direct influence in the total path length. Finally, control groups without exposure to head impacts, with and without athletic training, would allow for further interpretation. This may be particularly relevant for spinal cord excitability measurements, as results from the present study were compared with a non-matched control group.
Conclusion
When performed using a low-cost balance board, tandem-leg stance on an unstable surface and double-leg stance on a firm surface revealed relevant information on balance deficits following head impacts over a rugby season, even without diagnosed concussion. Spinal cord excitability should be further investigated in contact-sports population.
Acknowledgments
All authors thank the varsity female rugby team from the University of Victoria. RAM was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq;406917/2016–7); Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF; 193.001.010/2015) (FAPDF; 193.001.655/2017); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) –Finance Code 001.
References
Footnotes
SEB and BF contributed equally.
Contributors SEB was involved in the study design, data collection and data analysis; BF was involved in data analysis and interpretation, and manuscript preparation; RAM participated in data collection and analysis; GEPP and YS contributed to study design, data analysis and interpretation; EPZ participated in the study design, interpretation and manuscript preparation.
Funding This work was partially supported by an NSERC Discovery grant to EPZ.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This project was approved by the University of Victoria Human Research Ethics Committee (protocol #15–193).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Original data may be available upon reasonable request.