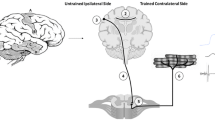
Abstract
The present review proposes the untested hypothesis that cross-education performed with a mirror increases the transfer of motor function to the resting limb compared with standard cross-education interventions without a mirror. The hypothesis is based on neuroanatomical evidence suggesting an overlap in activated brain areas when a unilateral motor task is performed with and without a mirror in the context of cross-education of the upper extremities. The review shows that the mirror-neuron system (MNS), connecting sensory neurons responding to visual properties of an observed action and motor neurons that discharge action potentials during the execution of a similar action, has the potential to enhance cross-education. After a literature search we narrowed the review to studies that examined healthy young adults who performed unilateral strength training and unilateral motor tasks with or without a mirror and assessed outcome measures in relation to the changes in brain activity, motor cortical excitability, and corticospinal excitability. We identified six chronic studies that examined the effects of unilateral strength training on neural adaptations and 15 cross-sectional studies that examined acute changes in brain activation, motor cortical and corticospinal excitability using imaging, electroencephalographic, magnetoencephalographic, and magnetic brain stimulation. There were two chronic and nine cross-sectional studies in which participants performed unilateral motor tasks while viewing the image of the active hand superimposed on the resting hand’s image. Collectively, the data suggest that the MNS is involved in cross-education and the hypothesis is tenable. However, future studies are needed to elucidate the precise mechanism of how the use of a mirror in a cross-education study augments transfer to the non-exercised limb. Recent studies show a strength-sparing effect in the immobilized arm after strength training of the free arm in healthy individuals, and improved bilateral function after unilateral exercise therapy in stroke patients. It is thus conceptually justified to conduct randomized clinical trials that supplement cross-education protocols with a mirror. Such a treatment could reduce muscle weakness caused by limb fractures, anterior-cruciate ligament reconstruction surgery, stroke, and other unilateral motor dysfunctions.






Similar content being viewed by others
References
Piaget J. Play, dreams, and imitation in childhood. London: Routledge; 1951.
Rizzolatti G, Fadiga L, Fogassi L, Gallese V. Resonance behaviors and mirror neurons. Arch Ital Biol. 1999;137(2–3):85–100.
Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 2005;15(6):632–7.
Heyes C. Where do mirror neurons come from? Neurosci Biobehav Rev. 2010;34(4):575–83.
Ray E, Heyes C. Imitation in infancy: the wealth of the stimulus. Dev Sci. 2011;14(1):92–105.
Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996;111(2):246–52.
Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–8.
Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci USA. 2001;98(24):13995–9.
Farthing JP, Borowsky R, Chilibeck PD, Binsted G, Sarty GE. Neuro-physiological adaptations associated with cross-education of strength. Brain Topogr. 2007 Winter;20(2):77–8.
Hortobágyi T. Cross education and the human central nervous system. IEEE Eng Med Biol Mag. 2005;24(1):22–8.
Zhou S. Chronic neural adaptations to unilateral exercise: mechanisms of cross education. Exerc Sport Sci Rev. 2000;28(4):177–84.
Munn J, Herbert RD, Gandevia SC. Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol. 2004;96(5):1861–6.
Hortobágyi T, Richardson SP, Lomarev M, Shamim E, Meunier S, Russman H, et al. Interhemispheric plasticity in humans. Med Sci Sports Exerc. 2011;43(7):1188–99.
Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol. 2006;101(5):1514–22.
Howatson G, Taylor MB, Rider P, Motawar BR, McNally MP, Solnik S, et al. Ipsilateral motor cortical responses to TMS during lengthening and shortening of the contralateral wrist flexors. Eur J Neurosci. 2011;33(5):978–90.
Ruddy KL, Carson RG. Neural pathways mediating cross education of motor function. Front Hum Neurosci. 2013;29(7):397.
Howatson G, Zult T, Farthing JP, Zijdewind I, Hortobágyi T. Mirror training to augment cross-education during resistance training: a hypothesis. Front Hum Neurosci. 2013;24(7):396.
Farthing JP, Krentz JR, Magnus CR, Barss TS, Lanovaz JL, Cummine J, et al. Changes in functional magnetic resonance imaging cortical activation with cross education to an immobilized limb. Med Sci Sports Exerc. 2011;43(8):1394–405.
Matthys K, Smits M, Van der Geest JN, Van der Lugt A, Seurinck R, Stam HJ, et al. Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study. Arch Phys Med Rehabil. 2009;90(4):675–81.
Nojima I, Mima T, Koganemaru S, Thabit MN, Fukuyama H, Kawamata T. Human motor plasticity induced by mirror visual feedback. J Neurosci. 2012;32(4):1293–300.
Thieme H, Mehrholz J, Pohl M, Behrens J, Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst Rev. 2012;(3):CD008449.
Bowering KJ, O’Connell NE, Tabor A, Catley MJ, Leake HB, Moseley GL, et al. The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. J Pain. 2013;14(1):3–13.
Läppchen CH, Ringer T, Blessin J, Seidel G, Grieshammer S, Lange R, et al. Optical illusion alters M1 excitability after mirror therapy: a TMS study. J Neurophysiol. 2012;108(10):2857–61.
Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609.
Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3(2):131–41.
Buccino G, Binkofski F, Riggio L. The mirror neuron system and action recognition. Brain Lang. 2004;89(2):370–6.
Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92.
Cattaneo L, Rizzolatti G. The mirror neuron system. Arch Neurol. 2009;66(5):557–60.
Small SL, Buccino G, Solodkin A. The mirror neuron system and treatment of stroke. Dev Psychobiol. 2012;54(3):293–310.
Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14(1 Pt 2):S103–9.
Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–67.
Grezes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia. 2002;40(2):212–22.
Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36(1):341–9.
Munzert J, Lorey B, Zentgraf K. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Res Rev. 2009;60(2):306–26.
Brighina F, La Bua V, Oliveri M, Piazza A, Fierro B. Magnetic stimulation study during observation of motor tasks. J Neurol Sci. 2000;174(2):122–6.
Clark DJ, Patten C. Eccentric versus concentric resistance training to enhance neuromuscular activation and walking speed following stroke. Neurorehabil Neural Repair. 2013;27(4):335-44.
Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73(6):2608–11.
Patuzzo S, Fiaschi A, Manganotti P. Modulation of motor cortex excitability in the left hemisphere during action observation: a single- and paired-pulse transcranial magnetic stimulation study of self- and non-self-action observation. Neuropsychologia. 2003;41(9):1272–8.
Roosink M, Zijdewind I. Corticospinal excitability during observation and imagery of simple and complex hand tasks: implications for motor rehabilitation. Behav Brain Res. 2010;213(1):35–41.
Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999;9(2):161–7.
Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, et al. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42(2):323–34.
Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–60.
Imamizu H, Kuroda T, Yoshioka T, Kawato M. Functional magnetic resonance imaging examination of two modular architectures for switching multiple internal models. J Neurosci. 2004;24(5):1173–81.
Sutbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88(5):555–9.
Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature. 1995;377(6549):489–90.
Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol. 1999;81(1):383–7.
Kristeva R, Keller E, Deecke L, Kornhuber HH. Cerebral potentials preceding unilateral and simultaneous bilateral finger movements. Electroencephalogr Clin Neurophysiol. 1979;47(2):229–38.
Newton J, Sunderland A, Butterworth SE, Peters AM, Peck KK, Gowland PA. A pilot study of event-related functional magnetic resonance imaging of monitored wrist movements in patients with partial recovery. Stroke. 2002;33(12):2881–7.
Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res. 2006;175(3):526–35.
Lee M, Gandevia SC, Carroll TJ. Unilateral strength training increases voluntary activation of the opposite untrained limb. Clin Neurophysiol. 2009;120(4):802–8.
Schulte T, Muller-Oehring EM. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol Rev. 2010;20(2):174–90.
Tominaga W, Matsubayashi J, Furuya M, Matsuhashi M, Mima T, Fukuyama H, et al. Asymmetric activation of the primary motor cortex during observation of a mirror reflection of a hand. PLoS One. 2011;6(11):e28226.
Carson RG, Ruddy KL. Vision modulates corticospinal suppression in a functionally specific manner during movement of the opposite limb. J Neurosci. 2012;32(2):646–52.
Arevalo AL, Baldo JV, Dronkers NF. What do brain lesions tell us about theories of embodied semantics and the human mirror neuron system? Cortex. 2012;48(2):242–54.
Catmur C, Gillmeister H, Bird G, Liepelt R, Brass M, Heyes C. Through the looking glass: counter-mirror activation following incompatible sensorimotor learning. Eur J Neurosci. 2008;28(6):1208–15.
Yavuzer G, Selles R, Sezer N, Sutbeyaz S, Bussmann JB, Koseoglu F, et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89(3):393–8.
Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263(1369):377–86.
Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res. 2005;163(1):118–22.
Rosen B, Lundborg G. Training with a mirror in rehabilitation of the hand. Scand J Plast Reconstr Surg Hand Surg. 2005;39(2):104–8.
Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–41.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21.
Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex. 2010;20(1):34–45.
Foltys H, Meister IG, Weidemann J, Sparing R, Thron A, Willmes K, et al. Power grip disinhibits the ipsilateral sensorimotor cortex: a TMS and fMRI study. Neuroimage. 2003;19(2 Pt 1):332–40.
Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111(2):344–9.
Hoy KE, Georgiou-Karistianis N, Laycock R, Fitzgerald PB. Using transcranial magnetic stimulation to investigate the cortical origins of motor overflow: a study in schizophrenia and healthy controls. Psychol Med. 2007;37(4):583–94.
Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28(22):5631–40.
Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89(3):1256–64.
Hortobágyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol. 2003;90(4):2451–9.
Dragert K, Zehr EP. Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp Brain Res. 2011;208(2):217–27.
Bologna M, Caronni A, Berardelli A, Rothwell JC. Practice-related reduction of electromyographic mirroring activity depends on basal levels of interhemispheric inhibition. Eur J Neurosci. 2012;36(12):3749–57.
Lee M, Hinder MR, Gandevia SC, Carroll TJ. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol. 2010;588(Pt 1):201–12.
Duchateau J, Hortobágyi T, Enoka RM. Acute and long-term neural adaptations to training. In: Gollhofer A, Taube W, Nielsen JB, editors. Motor control and learning. London: Routledge; 2012. p. 319–50.
Hubers A, Orekhov Y, Ziemann U. Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity. Eur J Neurosci. 2008;28(2):364–71.
Hortobágyi T, Lambert NJ, Hill JP. Greater cross education following training with muscle lengthening than shortening. Med Sci Sports Exerc. 1997;29(1):107–12.
Cincotta M, Borgheresi A, Balestrieri F, Giovannelli F, Rossi S, Ragazzoni A, et al. Involvement of the human dorsal premotor cortex in unimanual motor control: an interference approach using transcranial magnetic stimulation. Neurosci Lett. 2004;367(2):189–93.
Giovannelli F, Borgheresi A, Balestrieri F, Ragazzoni A, Zaccara G, Cincotta M, et al. Role of the right dorsal premotor cortex in “physiological” mirror EMG activity. Exp Brain Res. 2006;175(4):633–40.
Kidgell DJ, Stokes MA, Pearce AJ. Strength training of one limb increases corticomotor excitability projecting to the contralateral homologous limb. Motor Control. 2011;15(2):247–66.
Pearce AJ, Hendy A, Bowen WA, Kidgell DJ. Corticospinal adaptations and strength maintenance in the immobilized arm following 3 weeks unilateral strength training. Scand J Med Sci Sports. doi: 10.1111/j.1600-0838.2012.01453.x. [Epub ahead of print].
Fukumura K, Sugawara K, Tanabe S, Ushiba J, Tomita Y. Influence of mirror therapy on human motor cortex. Int J Neurosci. 2007;117(7):1039–48.
Hamzei F, Läppchen CH, Glauche V, Mader I, Rijntjes M, Weiller C. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair. 2012;26(5):484–96.
Funase K, Tabira T, Higashi T, Liang N, Kasai T. Increased corticospinal excitability during direct observation of self-movement and indirect observation with a mirror box. Neurosci Lett. 2007;419(2):108–12.
Shinoura N, Suzuki Y, Watanabe Y, Yamada R, Tabei Y, Saito K, et al. Mirror therapy activates outside of cerebellum and ipsilateral M1. NeuroRehabilitation. 2008;23(3):245–52.
Tominaga W, Matsubayashi J, Deguchi Y, Minami C, Kinai T, Nakamura M, et al. A mirror reflection of a hand modulates stimulus-induced 20-Hz activity. Neuroimage. 2009;46(2):500–4.
Farthing JP. Cross-education of strength depends on limb dominance: implications for theory and application. Exerc Sport Sci Rev. 2009;37(4):179–87.
Catmur C, Walsh V, Heyes C. Associative sequence learning: the role of experience in the development of imitation and the mirror system. Philos Trans R Soc Lond B Biol Sci. 2009;364(1528):2369–80.
Shin HW, Sohn YH. Interhemispheric transfer of paired associative stimulation-induced plasticity in the human motor cortex. Neuroreport. 2011;22(4):166–70.
Stromberg BV. Contralateral therapy in upper extremity rehabilitation. Am J Phys Med. 1986;65(3):135–43.
Stromberg BV. Influence of cross-education training in postoperative hand therapy. South Med J. 1988;81(8):989–91.
Farthing JP, Krentz JR, Magnus CR. Strength training the free limb attenuates strength loss during unilateral immobilization. J Appl Physiol. 2009;106(3):830–6.
Magnus CR, Arnold CM, Johnston G, Dal-Bello Haas V, Basran J, Krentz JR, et al. Cross-education for improving strength and mobility after distal radius fractures: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94(7):1247-55.
Ausenda C, Carnovali M. Transfer of motor skill learning from the healthy hand to the paretic hand in stroke patients: a randomized controlled trial. Eur J Phys Rehabil Med. 2011;47(3):417–25.
Michielsen ME, Selles RW, van der Geest JN, Eckhardt M, Yavuzer G, Stam HJ, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil Neural Repair. 2011;25(3):223–33.
Dragert K, Zehr EP. High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp Brain Res. 2013;225(1):93-104.
Papandreou M, Billis E, Papathanasiou G, Spyropoulos P, Papaioannou N. Cross-exercise on quadriceps deficit after ACL reconstruction. J Knee Surg. 2013;26(1):51–8.
Papandreou MG, Billis EV, Antonogiannakis EM, Papaioannou NA. Effect of cross exercise on quadriceps acceleration reaction time and subjective scores (Lysholm questionnaire) following anterior cruciate ligament reconstruction. J Orthop Surg Res. 2009; 4:2. doi:10.1186/1749-799X-4-2.
Acknowledgments
Jonathan Farthing was supported by sabbatical research travel funding from the University of Saskatchewan to visit the University of Groningen. No further sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review. The authors thank the reviewers for the detailed and insightful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
40279_2013_105_MOESM1_ESM.doc
Table S1. Characteristics of the reviewed studies that assessed cross-education and neural adaptations after unilateral strength training (top six studies) and studies that assessed cortical activity, motor cortical excitability, or corticospinal excitability during unilateral strength exercises (bottom 15 studies). (DOC 116 kb)
40279_2013_105_MOESM2_ESM.doc
Table S2. Characteristics of the reviewed studies that assessed cross-education and cortical adaptations after chronic mirror training (top two studies) and studies that assessed cortical activity, motor cortical excitability, or corticospinal excitability during acute mirror training (bottom nine studies). (DOC 69 kb)
Rights and permissions
About this article
Cite this article
Zult, T., Howatson, G., Kádár, E.E. et al. Role of the Mirror-Neuron System in Cross-Education. Sports Med 44, 159–178 (2014). https://doi.org/10.1007/s40279-013-0105-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-013-0105-2