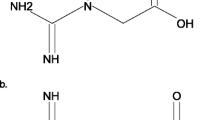
Abstract
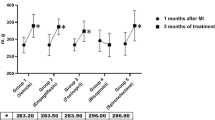
Physical exercise causes transient albuminuria. The mechanisms of postexercise albuminuria are not fully clarified but stimulation of the reninangiotensin system (RAS) probably plays a major role through intrarenal haemodynamic changes causing an elevated filtration pressure. In a randomised, double-blind, crossover study we compared the effects on urinary albumin excretion (UAE) of lisinopril (L) and atenolol (A) therapy, i.e. we aimed to investigate whether inhibition of the RAS or inhibition of β1-adrenoceptor-mediated effects of the sympathetic nervous system differed with regard to changes in UAE. Sixteen patients with uncomplicated primary hypertension were studied. Four standardised bicycle ergometer exercise tests were performed, before and after each active treatment period. UAE 30 min postexercise, determined by radioimmunoassay, was significantly lowered by both treatments: -278 μg·min-1 (L) and-199 μg·min-1 (A). The reduction of postexercise UAE achieved by treatment with the angiotensin-converting enzyme (ACE) inhibitor (L) was significantly greater than that achieved by the β1-selective adrenoceptor blocker treatment. Blood pressure (BP) at rest and during exercise were equally reduced by both drugs. In conclusion, this study showed that antihypertensive treatment with an ACE inhibitor was more effective in reducing exercise-induced albuminuria than a β1-selective adrenoceptor-blocking agent with a similar degree of BP reduction in patients with uncomplicated primary hypertension. This suggests that the RAS plays a major role in postexercise albuminuria in hypertensive subjects. The clinical significance of this finding, however, remains to be clarified.
Similar content being viewed by others
References
Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A (1989) Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32:219–226.
Shearman CP, Gosling P (1988) Microalbuminuria and vascular permeability. Lancet II:906–907.
Rambauske M, Fliser D, Ritz E (1992) Albuminuria of hypertensive patients. Clin Nephrol 38 [Suppl 1]:S40-S45.
Samuelsson O, Hedner T, Ljungman S, Herlitz H, Widgren B, Pennert K (1992) A comparative study of lisinopril and atenolol on low degree urinary albumin excretion, renal function and haemodynamics in uncomplicated, primary hypertension. Eur J Clin Pharmacol 43:469–475.
Kannel WB, Stampfer MJ, Castelli WP, Vester J (1984) The prognostic significance of proteinuria: the Framingham study. Am Heart J 108:1347–1352.
Samuelsson O (1988) Proteinuria as a prognostic factor during long-term antihypertensive care. Drugs 35 [Suppl 5]:48–54.
Mogensen CE (1984) Microalbuminuria predicts clinical proteinurina and early mortality in maturity-onset diabetes. N Engl J Med 310:356–360.
Mattock MB, Keen H, Viberti GC, et al (1988) Coronary heart disease and urinary albumin excretion rate in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 31:82–87.
Yudkin JS, Forrest RD, Jackson CA (1988) Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Lancet II:530–533.
Ljungman S (1990) The role of microalbuminuria in essential hypertension. Am J Hypertens 3:956–960.
Damsgaard EM, Froland A, Jørgensen OD, Mogensen CE (1992) Eight to nine years' mortality in known non-insulin dependent diabetics and controls. Kidney Int 41:731–735.
de Jong PE, Anderson S, de Zeeuw D (1993) Glomerular preload and afterload reduction as a tool to lower urinary protein leakage: will such treatments also help to improve renal function outcome? J Am Soc Nephrol 3:1333–1341.
de Venuto G, Andreotti C, Mattarei M, Pegoretti G (1985) Long-term captopril therapy at low doses reduces albumin excretion in patients with essential hypertension and no signs of renal impairment. J Hypertens 3 [Suppl 2]:143–145.
Poortmans JR, Rampaaer L, Wolfs J-C (1989) Renal protein excretion after exercise in man. Eur J Appl Physiol 58:476–480.
Esnault VLM, Poitron-Josse M, Testa A, Ginet JD, Le Carrer D, Guenel J (1991) Captopril but not acebutolol, prazosin or indomethacin decreases postexercise proteinuria. Nephron 58:437–442.
Christensen CK, Krusell LR (1987) Acute and long-term effect of antihypertensive treatment on exercise induced microalbuminuria in essential hypertension. J Clin Hypertens 3:704–712.
Romanelli G, Giustina A, Agabiti-Rosei E, Bossoni S, Girelli A, Muiesan ML, Moisen G, Giostina G (1989) Short-term effect of captopril and nifedipine on microalbuminuria induced by exercise in hypertensive diabetic patients. J Hypertens 6 [Suppl 6]:S312-S313.
Torffvit O, Castenfors J, Agardh C-D (1991) A study of exercise-induced microalbuminuria in type I (insulin-dependent) diabetes mellitus. Scand J Urol Nephrol 25:39–43.
Gosling D, Beevers DG (1989) Urinary albumin excretion and blood pressure in the general population. Clin Sci 76:39–42.
Gianconi S, Levanti C, Fommei E, Innocenti F, Seghieri G, Palla L, Palombo C, Buione S (1989) Microalbuminuria and causal and ambulatory blood pressure monitoring in normotensives and in patients with borderline and mild essential hypertension. Am J Hypertens 2:259–261.
Montagna B, Buzio C, Calderini C, Quaretti P, Migone L (1983) Relationship of proteinuria and albuminuria to posture and urine collection period. Nephron 35:143–144.
West JN, Gosling P, Dimmitt SB, Littler WA (1991) Non-diabetic microalbuminuria in clinical practice and its relationship to posture, exercise and blood pressure. Clin Sci 81 3:373–377.
Pedersen EB, Mogensen CE, Larsen JS (1981) Effects of excretion of albumin and β2-microglobulin in young patients with mild essential hypertension without treatment and during long-term propranolol treatment. Scand J Lab Invest 41:493–498.
Yamada T, Iscihara M, Ishikawa K, Hiramato K (1980) Protenuria and renal function during antihypertensive treatment for essential hypertension. J Am Geriatr Soc 28:114–117.
Parving HH, Jensen HA, Westrup M (1977) Increased transcapillary escape rate of albumin and IgG in essential hypertension. Scand J Lab Invest 37:223–227.
Marre M, LeBlanc H, Suarez L, Guyenne TT, Menard J, Passa P (1987) Converting enzyme inhibition and kidney function in normotensive diabetic patients with persistent microalbuminuria. BMJ 294:1448–1452.
Andersen S, Rennke HG, Brenner BM (1986) Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest 77:1993–2000.
Jackson B, Johnston C (1988) The contribution of systemic hypertension to progression of chronic renal failure in the rat remnant kidney: effect of treatment with an angiotensin converting enzyme inhibitor or a calcium inhibitor. J Hypertens 6:495–501.
Hallab M, Gallois Y, Chatellier G, Rohmer V, Fressinaud P, Marre M (1993) Comparison of reduction in microalbuminuria by enalapril and hydrochlorothiazide in normotensive patients with insulin dependent diabetes mellitus. BMJ 306:175–82.
Hall JE (1986) Regulation of glomerular filtration rate and sodium excretion by angiotensin II. Fed Proc 45:1431–1437.
Kanwar YS (1984) Biology of disease. Biophysiology of glomerular filtration and proteinuria. Lab Invest 51:7–21.
Brunner HR, Waeber B, Nussberger J (1987) Renal effects of converting enzyme inhibition. J Cardiovasc Pharmacol 9 [Suppl 3]:S6-S14.
Morelli E (1990) Effects of converting enzyme inhibition on barrier function in diabetic glomerulopathy. Diabetes 39:76–82.
Raij L, Keane WF (1985) Glomerular mesangium: its function and relationship to angiotensin II. Am J Med 79 [Suppl 3C]:24–30.
Castenfors J (1967) Renal function during exercise. Acta Physiol Scand 293(Suppl 70):1–44.
Staessen J, Fagard R, Hespel P, Lijnen P, Vanhmees L, Amery A (1987) Plasma renin system during exercise in normal men. J Appl Physiol 63:188–194.
Mittleman KD, Zambraski EJ (1992) Exercise-induced proteinuria is attenuated by indomethacin. Med Sci Sports Exerc 24:1069–1074.
Rudberg S, Sätterström G, Dahlqvist R, Dahlquist G (1993) Indomethacin but not metoprolol reduces exercise-induced albumin excretion rate in type I diabetic patients with microalbuminuria. Diab Med 10:460–464.
Viberti GC (1992) Introduction to a structural basis for renal and vascular complications in diabetes and hypertension. J Hypertens 10 [Suppl 1]:S1-S4.
Parving HH, Nielsen SL, Larsen NA (1974) Increased transcapillary escape rate of albumin, IgG, and IgM during angiotensin-II-induced hypertension in man. Scand J Lab Invest 34:111–118.
Dzau VJ (1989) Multiple pathways of angiotensin production in the blood vessel wall: evidence, possibilities and hypothesis. J Hypertens 7:933–936.
Unger T, Gohlke P (1990) Tissue renin-angiotensin systems in the heart and vasculature: possible involvement in the cardiovascular actions of converting enzyme inhibitors. Am J Cardiol 65:3I–10I.
Chobanian AV, Handenschild CC, Nickerson C, Drago R (1990) Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension 15:327–331.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
R»ngemark, C., Hedner, T., Samuelsson, O. et al. Lisinopril reduces postexercise albuminuria more effectively than atenolol in primary hypertension. Eur J Clin Pharmacol 49, 267–271 (1996). https://doi.org/10.1007/BF00226326
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00226326