Article Text
Abstract
Introduction Anti-neoplastic treatment is synonymous with an inactive daily life for a substantial number of patients. It remains unclear what is the optimal setting, dosage and combination of exercise and health promoting components that best facilitate patient adherence and symptom management in order to support cardio-respiratory fitness and lifestyle changes in an at-risk population of pre-illness physically inactive cancer patients.
Methods Patients with breast or colon cancer referred to adjuvant chemotherapy and by the oncologists pre-screening verified as physically inactive were eligible to enter a randomised three-armed feasibility study comparing a 12-week supervised hospital-based moderate to high intensity exercise intervention or alternate an instructive home-based12-week pedometer intervention, with usual care.
Results Using a recommendation based physical activity screening instrument in order to correspond with cardio-respiratory fitness (VO2 peak) proved to be an applicable method to identify pre-illness physically inactive breast and colon cancer patients. The study demonstrated convincing recruitment (67%), safety and intervention adherence among breast cancer patients; while the attendance rate for colon cancer patients was notably lower (33%). VO2-peak declined on average 12% across study groups from baseline to 12 weeks though indices towards sustaining watt performance and reduce fat mass favoured the hospital-based intervention. Pedometer use was well adapted in both breast and colon cancer patients.
Conclusions Despite a fair adherence and safety, the current study calls into question whether aerobic exercise, regardless of intensity, is able to increase VO2-peak during texane-based chemotherapy in combination with Neulasta in physically inactive breast cancer patients.
Trial Registration: ISRCTN24901641
- Cancer
- Sports & exercise medicine
- Rehabilitation
- Feasibility
- Research
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Strengths and limitations of this study
The feasibility study demonstrated that prediagnostic physically inactive patients with breast or colon cancer may be identified by clinicians by using a simple screening instrument based on national recommendations for physical activity that associates with low cardiorespiratory capacity at onset of adjuvant chemotherapy.
Physically inactive patients with breast cancer may be motivated to participate in supervised comprehensive or home-based exercise interventions of moderate-to-high intensity at onset of adjuvant chemotherapy. The low recruitment and high attrition of patients with colon cancer made it inadequate to raise a clear conclusion on feasibility.
Both interventions were well timed and showed fair adherence and safety among patients with breast cancer but were partly inconclusive for patients with colon cancer regarding timing and volume of exercise components.
The current feasibility study calls into question whether aerobic exercise, regardless of intensity, is able to increase cardiorespiratory capacity during taxane-based chemotherapy in combination with Neulasta among patients with breast cancer.
Background
In Denmark, 4637 people were diagnosed with breast cancer and 2551 with colon cancer during 2011.1 Improved treatment has increased the expected 5-year survival rate to 79% for breast cancer and 52% for colon cancer.1 ,2 A European survey among cancer survivors reported recently that <25% meet the current physical activity guidelines.3 Studies on exercise oncology are predominantly performed following chemotherapy and few studies involve patients with colorectal cancer.4–7 Of relevance, Courneya et al8 found that symptoms and side effects from chemotherapy are dominant barriers to attending exercise sessions among survivors of breast cancer.
Regular leisure time physical activity among patients with breast or colon cancer may reduce the incidence and risk of relapse.9–12 Other studies have found an elevated prevalence of predisposing lifestyle factors (weight gain, hypertension, metabolic dysfunction, physical inactivity, smoking) and an increased risk of developing heart disease among patients with cancer.13 ,14 These findings necessitate the integration of lifestyle modifications in oncology rehabilitation15–18 and the promotion of increased physical activity specifically for physically inactive or sedentary cancer survivors. A review by Wahnefried et al,19 “Riding the crest of the teachable moment”, suggests that cancer survivors spontaneously adopt lifestyle changes in the hope of improving their health. A few clinical studies have documented this tendency towards lifestyle change,20 but others have failed to confirm it.21 ,22 It remains unclear, though, what the optimal setting, timing during cancer treatment and survivorship, dosage and combination of exercise and health-promoting components best facilitate patient adherence and symptom management to support physiological improvements and sustainable lifestyle changes in this at-risk physically inactive cancer population. In general, there is a lack of powerful exercise studies examining physically inactive or sedentary cancer populations and during chemotherapy in particular.3
The objective of the present study is to investigate the capability of oncologists and nurses to evaluate physical activity among patients with breast or colon cancer during adjuvant chemotherapy and to recruit physical inactive patients for exercise intervention. The feasibility study examines adherence to one of two multimodal exercise interventions lasting 12 weeks, a hospital based, high intensity, group exercise intervention, and a home based, low intensity, individual pedometer intervention compared to a randomly selected control group and by targeting cardiorespiratory fitness (peak oxygen consumption; VO2 peak) as the primary outcome of interest.
Methods
Participants
Inclusion: Patients with breast or colon cancer referred to adjuvant chemotherapy, performance status 0–1 and verified during prescreening by oncologists or nurses as being physically inactive using guidelines from the Danish Health and Medicines Authority (150 min of regular and moderate recreational physical activity and at least 2×20 min of strenuous exercise per week)23 were eligible. A clinical nurse specialist informed patients in depth about the study's rationale, intervention and the scientific tests to be conducted. The study protocol contains additional details on eligibility.24
Exclusion: Patients with symptomatic heart disease (angina pectoris, acute coronary syndrome) within the past 6 months, and patients who were unable to read and understand Danish, were not eligible to enter the study.
Ethics
All patients provided informed written consent before entering the study. The Scientific Committee of the Capital Region (file no. H-1-2011-131) and the Danish Data Protection Agency (file no. 2011-41-6349) approved the study. Trial registration: Current Controlled Trials ISRCTN24901641.
Study design
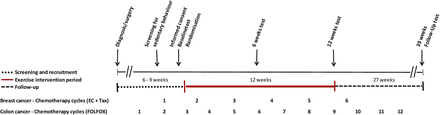
The study was designed as a randomised controlled, three-armed feasibility study comparing a 12-week supervised, hospital-based moderate-to-high exercise intervention and a non-supervised instructive 12-week pedometer intervention with usual care (figure 1). The randomised controlled trial (RCT) design was chosen in order to examine barriers for recruitment, adherence, safety aspects and potential efficacy related to study group allocation. The scope of the feasibility study was not designed, however, to investigate significant effects in outcomes between groups.
Global overview of study evaluation during chemotherapy (EC, epirubicin and cyclophosphamid FOLFOX, oxaliplatin and 5-FU (5-fluorouracil) and folinic acid; Tax, taxotere).
Following baseline testing, patients were sequentially numbered, stratified by diagnosis and randomised (equal weight 1:1:1) by computer at the Copenhagen Trial Unit (CTU). To test feasibility, the goal was to include 45 patients undergoing adjuvant chemotherapy.
Setting
The present project was conducted at the Department of Oncology, Copenhagen University Hospital, Rigshospitalet and at the Center for Integrated Rehabilitation of Cancer Patients (CIRE), Copenhagen, Denmark, established and supported by the Danish Cancer Society and the Novo Nordic Foundation. CIRE adheres to three key intervention principles: (1) Early initiation of an intervention during cancer treatment; (2) EXercise/physical activity and (3) Patient ACTivation (EEX-ACT).25 ,26
Intervention
Study arm 1 Supervised hospital-based group exercise intervention+health promotion counselling and symptom management (HIGH HOSP) (6 weeks; 9 h/week+6 weeks; 6 h/week)
Patients were offered a 12-week supervised exercise programme in groups of 10–14 patients by an exercise physiologist and a clinical nurse specialist (table 1). The first 6 weeks (part I, 9 h/week) included three training sessions per week comprising high-intensity/low-intensity components (cardiorespiratory training on stationary bikes, resistance training, relaxation training and massage), as well as one restorative session ‘Body awareness’ per week. The total training volume corresponded to approximately 43 metabolic equivalent of task (MET) hours per week.27 An all-sports training element that included ball games, dance and circuit training was introduced during the last 6 weeks of the intervention (part II, 6 h/week) with a total training volume of 40 MET hours/week. Furthermore, patients received individual health promotion counselling and symptom management at baseline and at 6, 12 and 39 weeks. Pre-exercise screening took place before each session that involved moderate-to-high-intensity physical training27 ,28 (see study protocol24).
Hospital-based supervised group exercise intervention
Study arm 2 Home-based individual progressive pedometer intervention, health promotion counselling and symptom management (LOW PED)
The pedometer programme was individually organised and designed to progressively support increased physical activity during adjuvant chemotherapy (table 2). All pedometer data were delivered electronically by connecting the pedometers to Omron Health Management Software uploaded in study investigators’ work station computers.
Home-based individual progressive pedometer intervention
The individual pedometer instruction was provided by a clinical nurse specialist in cancer and exercise. Patients were encouraged to enhance their physical activity levels and to avoid physical inactivity by integrating exercise into activities of daily living during chemotherapy. The overall goal was to achieve a low/moderate recreational physical activity level of 30 min/day and ultimately 10 000 steps/day, five times per week.29 To enhance adherence in wearing and using pedometers, patients were instructed and supported with a tighter schedule at the beginning than at the end of the intervention. The patients were (1) issued an Omron Walking Style Pro pedometer with PC access capability that made it possible to visualise the patient's exercise achievements on a daily, weekly and monthly basis, as well as scheduled instruction and evaluation at baseline and at weeks 2, 4, 6, 9 and 12; (2) received similar individual face-to-face health promotion counselling as the HIGH HOSP group, including clinical advice concerning symptom management at baseline and at weeks 6 and 12 and later on at 39 weeks (see study protocol24).
Study arm 3: CONTROL
The control group received standard care with no specific restrictions on participation in physical activity. Owing to ethical considerations and growing scientific evidence, control patients were in fact motivated and advised by their clinicians to be physically active.27 ,30 Following the control period, patients were offered participation in body and cancer,27 an exercise programme provided by the Copenhagen Region hospitals after the 12-week study period.
Outcome measures
In accordance with the study protocol,24 the primary outcome cardiorespiratory fitness/VO2 peak and secondary physiological and patient-reported outcomes (PRO) were measured at baseline (inclusion) and at 6 and 12 weeks (end of intervention).
Primary outcome: Cardiorespiratory fitness measured as the VO2 peak and determined by an incremental test on a cycle ergometer (Monark Ergomedic 839E) and direct measures of respiratory gases.
Secondary outcomes: Physiological measures (respiratory exchange ratio (RER), maximum heart rate (HRmax), spirometry, test haemoglobin, fasting full body dual-energy X-ray absorptiometry (DXA) scan, digital pedometer steps, aerobic walking time and PRO, including the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ) C-30, the 36-Item Short Form (SF-36), the Hospital Anxiety and Depression Scale (HADS) and a supplemental questionnaire (physical activity/categorical, labour, disability, participation in local rehabilitation lifestyle factors (eg, smoking cessation, alcohol, physical activity).24
An oncology nurse specialist or project physiotherapist entered physiological variables, questionnaires and medical data gathered from patients’ medical records into a database, OpenClinica, hosted by CTU, who exclusively had access to unblinded data while the trial was being conducted.
Statistics and analytic plan
The principal analysis employed the intention-to-treat approach. The explorative aspect includes descriptive statistical analysis across study groups to provide insight into mean values, SDs and the potential application of objective measurement tools and standardised PRO instruments.
Results
Patient characteristics and feasibility analyses
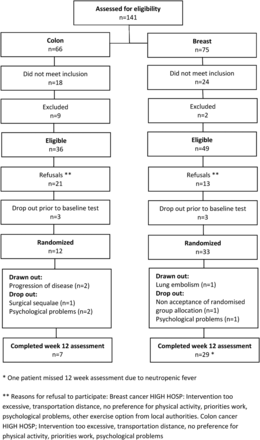
A total of 141 patients were assessed for eligibility, and this resulted in 45 patients being included, equalling an acceptance rate of 67% for breast cancers and 33% for colon cancers (figure 2). The number of refusals among patients with colon cancer was considerably high (58%). Compared with breast cancer, patients with colon cancer was older, had more postsurgical complaints (eg, prolonged tissue healing, ostomies and bowel problems) and accordingly were less prepared for a tight intervention programme schedule (9 h/week) during adjuvant chemotherapy. Six patients (3 with breast cancer and 3 with colon cancer) dropped out prior to the baseline test, mainly due to concerns about the level and amount of exercise in the hospital-based intervention regarding severe side effects, non-control of ostomy output and sequelae from surgery.
Flow chart.
Table 3 presents the study population characteristics. All patients received adjuvant chemotherapy prior to and during the 12-week intervention or control period. On average, patients with breast cancer had received 1,3 chemotherapy cycles prior to study inclusion and patients with colon cancer 3,2 cycles supporting the feasibility of timing rehabilitation at this specific time point during the initial treatment. The time since diagnosis to baseline test was on average 77 days with an SD of 32. The majority 89% (n=40) had primary surgery within 0–20 days from the date of their initial diagnosis, whereas 11% (n=5) of participants were going through surgery 37–133 days after their initial diagnosis was established.
Patient characteristics by study groups
Using two superior physical activity screening criteria from the Danish Health and Medicines Authority23 showed that not performing strenuous physical activity at least 20 min twice a week was the major patient-reported cause for study eligibility, whereas the majority (71%) reported an adequate performance of at least 150 min of moderate intensity recreational physical activity per week (figure 3). Accordingly, 67% fell into the low or very low group of VO2 peak values at baseline when comparing individual VO2 peak values with the Scandinavian background population.31
Maximal oxygen uptake peak oxygen consumption.
Adherence to the interventions and safety aspects: Thirty-seven of 45 patients (91% breast, 58% colon, respectively) completed the study given an attrition rate of 18%. Patients with breast/colon cancer adhered to the HIGH HOSP intervention in 74%/50% of the total expected training days and with a tendency of falling adherence during the second half of the observational period. Patients wore the pedometer (breast/colon) in 75%/81% of the total expected days, respectively. The safety issues taken into consideration preceding maximum physiological tests and at daily attendance for the HIGH HOSP intervention followed a standardised and implemented procedure described previously.27 No serious adverse reactions related to the interventions were reported. On eight occasions, adverse events were reported during the high-intensity exercise or pedometer programme that led to discontinuation due to discomfort, for example, pain, neuropathies, nausea/vomiting, fatigue, neutropenia, fever, diarrhoea. Two patients in the HIGH HOSP intervention discontinued intermittently due to hospitalisation (lung embolism, anal fissure and infection). One patient with breast cancer completed the 12-week HIGH HOSP intervention but missed the 12-week assessment due to neutropenic fever following taxane-based chemotherapy. The primary determinant for discontinuation in the pedometer group was severe taxane-induced pain (n=4) resulting in affected walking ability and decreasing the level of adherence in wearing pedometers.
Three patients were ‘drawn out’ by study investigators due to progression of underlying cancer (n=2) or lung embolism (n=1), while reasons for patients ‘drop out’ were sequelae from surgical complication (n=1); non-acceptance of randomised group allocation (n=1); and psychological discomfort at this early stage of treatment (n=3).
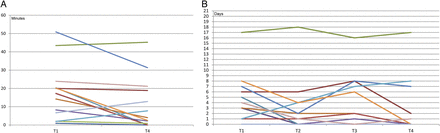
Pedometer usability: Table 4 shows achievements and patient usability with the pedometer divided into four measurement points, each covering 3 weeks (T1, T2, T3 and T4). No progression in average of total steps, aerobic walking time and days with >10 000 steps was observed during the intervention, though some heterogeneity was found between individuals (figure 4).
12 weeks pedometer achievements and adherence, n=14
Average aerobic walking time (3 weeks average) at baseline (T1) versus intervention completion (T4) (A) and number of days with 10 000 steps achieved in cycles of 21 days measured four times (T1–T4) (B).
Health-related outcomes
Physiological test validity of primary outcome: Cardiorespiratory fitness VO2 peak: The use of the gold standard for VO2 peak and direct measures of respiratory gases demonstrated high validity judged on the RER, perceived exertion (Borgs Rating of Perceived Exertion (RPE)) and percentage of HRmax at peak exercise testing. There was no indication of improvements or declines in test habituation/performance at test time points (table 5), which supports the test applicability when measuring peak performance in patients with breast or colon cancer receiving adjuvant chemotherapy.
VO2 peak test performance
Table 6 shows the changes in cardiopulmonary capacity and performance capacity. By using analysis of variance (one-way ANOVA) did the primary outcome measure, mean VO2 peak, decrease significantly within groups from baseline to postintervention (12 weeks) in breast cancer groups (high, low and control). There were minor changes within groups that potentially favoured the HIGH HOSP intervention in performance capacity (Watt max). Considering colon cancer, our tests suggested an improvement on the VO2 peak and Watt performances in all study groups.
Primary outcome VO2 peak
Table 7 shows muscle strength and results from the DXA scan at baseline and at weeks 6 and 12. In general, we found improvement in strength in all study groups. Results favoured the HIGH HOSP intervention by reducing fat mass and increasing lean body mass compared with LOW PED and Controls.
Muscle strength and body composition
Secondary outcomes: Selected results from PROs: Selected PRO findings are presented, primarily among breast cancers, due to the relatively small group of colon cancers in the study. Table 8 provides an overview of selected PRO scales based on the given mean values and SD. A full analysis of the PROs may be available in online supplementary material. The patient-reported instruments were generally applicable to the breast or colon cancer population in this pilot study. Ceiling effects occurred in EORTC and SF-36 in relation to the physical functioning scales, whereas emotional scales (emotional functioning on EORTC and role emotional on SF-36) showed the potential functional effects of interventions. These results were supported by findings on HADS, indicating that there was less anxiety related to the HIGH HOSP intervention. Notably, pain increased linearly on the EORTC from weeks 6 to 12, corresponding to the planned shift in the antineoplastic agent from cyclophosphamide to taxane. Sleeping problems and dyspnoea seemed to be of significant importance (see table 8 and the online supplementary material for the full EORTC and Medical Outcome Study SF-36 analyses).
PRO breast (selected scales/results)
Discussion
Identifying physically inactive or sedentary cancer remains controversial due to the inconsistency in methods (patient reported and/or physiological measurements) for defining this target population in question.23 ,32–36 A consensus definition of sedentary behaviour has not yet been established, although agreement exists that sedentary behaviour is not classified as all behaviours separated from moderate-to-vigorous physical activity. A recent systematic review by Bourke et al3 on interventions to improve exercise behaviour in sedentary cancer survivors defines the term sedentary as cancer survivors not meeting recommended physical activity guidelines. Others have defined sedentary behaviour as the amount of activity ≤1,5 METs.37 Based upon patient report at baseline 71% of participants did reach an activity level of approximately 30 min of light to moderate leisure time physical activity per day, which is equivalent to a MET intensity of 3,0.38 We did not measure the amount of sedentary time spent on a daily or weekly basis, which is why a classification of the participants as sedentary may be biased. However, none of the participants reported that they were doing vigorous physical activities prior to their cancer diagnosis, why physically inactive not meeting recommended guidelines seem to be the most appropriate term. Accordingly, this study bridges the gap and approves the feasibility of using national guidelines as a threshold for patient-reported low physical activity assessment using national recommendations and a corresponding low VO2 peak measure at baseline compared with the Scandinavian background population (figure 3).
Furthermore, our feasibility study demonstrated convincing recruitment, safety and intervention adherence among physically inactive patients with breast cancer at onset of adjuvant chemotherapy, while the attendance and acceptance rate for patients with colon cancer was notably lower and therefore insufficient to raise any clear conclusions for this subgroup. The major barriers for hindering attendance of patients with colon cancer involved the weekly volume and HIGH HOSP exercise components offered (9 h weekly) in relationship to the surgical sequelae the patients experienced and due to the higher frequency of hospitalisation and chemotherapy cycles. Consequently, the present study does not justify that the dose (volume) of exercise should be equal between these two physically inactive cancer populations and points to the need of exercise modifications for colon cancers in order to increase recruitment. Notably, five of seven patients with colon cancer who completed the 12 weeks test improved their VO2 peak during adjuvant chemotherapy and 12-week follow-up assessment. However, owing to the limited inclusion and higher attrition among patients with colon cancer, we focused on recruitment and adherence results irrespective of the remarkable physiological improvements for some relatively younger men with colon cancer across group assignment. The challenge of designing an appropriate exercise interventional programme and broadening recruitment of patients with colon cancer therefore remains unsolved and as reflected in the limited scientific exercise literature during adjuvant chemotherapy for this specific subgroup.3 ,4
Considering the included physically inactive patients with breast cancer, our findings correspond to a meta-analysis by Husebo et al39 predicting exercise adherence in moderate-to-vigorous programmes among cancer populations to vary between 42% and 92%. The test adherence of 84% is in line with the limited literature among screened physically inactive patients with breast cancer referred to exercise intervention during chemotherapy.40–42 We propose that the identification of patients and the oncologists’ recommendation of physical exercise at time of onset for adjuvant chemotherapy are suitably timed to co-create opportunities for facilitating recruitment among these sedentary subgroups.43 Moreover, we found that patient motivation and sustained participation may counteract the exercise barriers despite patients experiencing a range of escalating symptoms and side effects (fatigue, pain, sleeping problems and dyspnoea) from baseline to the 12-week assessment. However, severe symptoms and side effects decreased attendance in the interventions with, for example, perceived pain as the dominant cause affecting walking ability in the pedometer group. The landscape and experience of symptoms and side effects along with motivational factors need to be explored in larger RCT samples that allow stratification and subgroup analyses.
The clinical and public health rationale of promoting, enhancing and sustaining physical activity, especially among the physically inactive or sedentary risk populations, has pushed for the integration of practical, non-supervised interventions as the use of pedometers and accelerometers during treatment and cancer survivorship.44–53 Patients allocated to pedometer use were not able to increase the number of walking steps during the present intervention and had, mainly due to a progression in the experience of taxane-related pain, a tendency of falling adherence in wearing their pedometers at the end of the intervention. However, the lowest quartile of adherence is still within a level >70% as found in sufficient studies incorporated in a systematic Cochrane review of exercise studies for women receiving adjuvant therapy for breast cancer.54 Moreover, pedometer data included in our analysis solely comprise data from where pedometers were actually used. We assume that days on which patients did not wear their pedometers could reflect even lesser steps than on days with registered pedometer data. Our finding is in contrast to the majority of studies performed post chemotherapy44–53 and findings from a recent study by Backman et al44 2013 that found a high level of physical activity performance and goal achievement among a similar sedentary cancer population during adjuvant chemotherapy. We are unaware whether this discrepancy is due to pedometer measurement validity, the type and nature of the pedometer intervention or whether or not pedometer data are based on patient reports or electronically transferred to investigator computers.
On the basis of the existing evidence,4 ,27 ,55–57 we hypothesised that exercise in favour of moderate-to-vigorous intensity could increase the participants’ physical capacity (ie, VO2 peak, Watt performance, muscle strength and body composition).24 The uniform maximum values of RER and HRmax at baseline and at 6 and 12 weeks of testing indicate that the maximal incremental cycle ergometry test is reproducible and valid for determination of the VO2 peak in these specific sedentary cancer populations. The majority of patients reached the criteria for achieving a valid VO2 max (RER>1.15; HRmax>expected HRmax—10 bpm).
The primary outcome, cardiorespiratory fitness (VO2 peak), decreased significantly in study groups. In general, the use of test-blinded assessors, the application of an individualised incremental test protocol and the utilisation of gold standard methods for VO2 peak measurement58 ,59 minimise test error, lending credibility to the results. Nonetheless, the aforementioned observation raises some concerns regarding the cardiorespiratory training potential in the intervention groups. From a physiological perspective, the HIGH HOSP intervention, with the combined aerobic and resistance components, could be affected by the use of two different exercise modalities that may reduce the other's effect.60 The loss in VO2 peak of 2.1 mL/kg min after 12 weeks in the HIGH HOSP intervention, however, is comparable to a study by Courneya et al61 in which the intervention group received aerobic training at an identical aerobic volume and lower intensity rate.
Our observation that neither the HIGH HOSP nor the LOW PED group could reverse expected declines in the VO2 peak is in striking contrast to the prevailing assumption in previous evidence that aerobic exercise in patients with breast cancer during chemotherapy promotes significant gains in cardiorespiratory fitness.4 ,27 ,57 A recent review of observational studies and two large RCTs using gold standard methods for the determination of cardiorespiratory fitness reported that the VO2 peak decreases in patients with breast cancer during adjuvant chemotherapy.61–65 One possible explanation could be attributed to the use of taxane-based chemotherapy and is in line with two large RCTs.61 ,65 The causal relationship is unknown; however, we speculate that the muscular toxicity associated with taxane-based chemotherapy66 could reduce aerobic exercise intensity due to pain,67 thus leading to reductions in the cardiorespiratory response. There is not sufficient power in the present study to support this hypothesis. This central physiological question is explored in our ongoing larger trial, with the intention to clarify the possible harmful effects of adjuvant taxane-based chemotherapy on cardio-respiratory fitness and the potential preservative effects of aerobic exercise.
The HIGH HOSP group showed a positive response in Watt performance after 6 and 12 weeks and a tendency to experience the most favourable changes in body composition towards a higher proportion of lean body mass and a reduced proportion of fat mass. The higher proportion of lean mass was supported by gains in muscle strength. These favourable changes could potentially thwart expected increases in fat mass and reductions in lean mass in women undergoing adjuvant chemotherapy for breast cancer,68 potentially leading to decreases in the risk of premature death associated with increased fat mass in the long run.69
In summary
This study calls into question whether aerobic exercise, regardless of intensity, is able to increase cardiorespiratory capacity (VO2 peak) during taxane-based chemotherapy in combination with Neulasta.61 ,63 Conversely, the study does not show whether the decline in VO2 peak would have been greater without intervention due to the design, sample size, control group contamination and waiting list attendance.
The complexity of integrating exercise intervention within adjuvant chemotherapy for sedentary patients with breast cancer seems adequate in timing and dose (volume), while the comparative effects of different interventions are explored in an ongoing larger trial.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
- Data supplement 2 - Online supplement
Footnotes
Contributors All authors have contributed substantially to the creation and revision of the manuscript. TM was first author and had together with CL, CA, BE and LA a leading part during the writing process. KBC was responsible for study analyses. CL and KB had a leading role in the study coordination of tests and intervention activities. TM, CL, CA and KB were primarily responsible for delivering the interventions, PO performed, analysed and coordinated the DXA scan, LW obtained data from patient records and PRO's to the study database. BE, LN and UB screened and identified patients in the Oncology Clinic. TM and CA informed patients and obtained written informed consent.
Funding The research is supported by grants from the Center for Integrated Rehabilitation of Cancer patients (CIRE), which was established in 2011 and is supported by the Danish Cancer Society and the Novo Nordisk Foundation. The project has furthermore received grants from TrygFonden Denmark, grant number: 7-12-0401.
Competing interests None declared.
Ethics approval The Scientific Committee of the Capital Region (file no. H-1-2011-131) and the Danish Data Protection Agency (file no. 2011-41-6349).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.